Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Micheau, C.; Ueda, Yuki; Motokawa, Ryuhei; Akutsu, Kazuhiro*; Yamada, Norifumi*; Yamada, Masako*; Moussaoui, S. A.*; Makombe, E.*; Meyer, D.*; Berthon, L.*; et al.
Journal of Molecular Liquids, 401, p.124372_1 - 124372_12, 2024/05
Supramolecular organization of extractant molecules impacts metal ions separation behavior. Probing bulk and interfacial structures of the relevant systems is expected to provide key insights into the metal ion selectivity and kinetic aspects. The supramolecular features of two solvent extraction systems based on malonamide extractants THMA in toluene and DBMA in n-heptane were studied using small-angle X-ray scattering for the organic bulk phases, as well as interfacial tension and neutron reflectivity measurements for the interfaces. In the bulk solution, THMA forms dimeric/trimeric associates but no aggregates in toluene, while DBMA forms large aggregates in n-heptane. On the other hand, THMA accumulates in a diffuse layer at the interface at high THMA concentration, whereas DBMA forms a compact but thinner layer. After Pd(II) extraction, the thickness of interfacial layers decreases in the case of THMA, and totally vanishes in the case of DBMA. Based on these new structural information, two mechanisms are proposed for Pd(II) and Nd(III) extraction with malonamides. In toluene, THMA associates slightly accumulate in the vicinity of the interface, then coordinate Pd(II) and diffuse into the organic bulk phase. In n-heptane, DBMA aggregates adsorb at the interface then pick up Nd(III) cations in their polar cores and finally diffuse into the bulk.
 solution in the gigapascal pressure range
solution in the gigapascal pressure rangeYamaguchi, Toshio*; Fukuyama, Nami*; Yoshida, Koji*; Katayama, Yoshinori*; Machida, Shinichi*; Hattori, Takanori
Liquids, 3(3), p.288 - 302, 2023/09
We report the structure of an aqueous 2 mol/kg MgCl solution at pressures from 0.1 MPa to 4 GPa and temperatures from 300 to 500 K revealed by X-ray and neutron scattering measurements. The scattering data are analyzed by empirical potential structure refinement (EPSR) modeling to derive the pair distribution functions, coordination number distributions, angle distributions, and spatial density functions as a function of pressure and temperature. Mg
solution at pressures from 0.1 MPa to 4 GPa and temperatures from 300 to 500 K revealed by X-ray and neutron scattering measurements. The scattering data are analyzed by empirical potential structure refinement (EPSR) modeling to derive the pair distribution functions, coordination number distributions, angle distributions, and spatial density functions as a function of pressure and temperature. Mg forms rigid solvation shells extended to the third shell; the first solvation shell of six-fold octahedral coordination with about six water molecules at 0 GPa transforms into about five water molecules and one Cl
forms rigid solvation shells extended to the third shell; the first solvation shell of six-fold octahedral coordination with about six water molecules at 0 GPa transforms into about five water molecules and one Cl due to the formation of the contact ion pairs in the GPa pressure range. The Cl
due to the formation of the contact ion pairs in the GPa pressure range. The Cl solvation shows a substantial pressure dependence; the coordination number of a water oxygen atom around Cl
solvation shows a substantial pressure dependence; the coordination number of a water oxygen atom around Cl increases from 8 at 0.1 MPa/300 K to 10 at 4 GPa/500 K. The solvent water transforms the tetrahedral network structure at 0.1 MPa/300 K to a densely packed structure in the GPa pressure range; the number of water oxygen atoms around a central water molecule gradually increases from 4.6 at 0.1 MPa/298 K to 8.4 at 4 GPa/500 K.
increases from 8 at 0.1 MPa/300 K to 10 at 4 GPa/500 K. The solvent water transforms the tetrahedral network structure at 0.1 MPa/300 K to a densely packed structure in the GPa pressure range; the number of water oxygen atoms around a central water molecule gradually increases from 4.6 at 0.1 MPa/298 K to 8.4 at 4 GPa/500 K.
 and nano zero-valent iron
and nano zero-valent ironIslam, M. S.*; Maamoun, I.; Falyouna, O.*; Eljamal, O.*; Saha, B. B.*
Journal of Molecular Liquids, 370, p.121005_1 - 121005_11, 2023/01
Times Cited Count:14 Percentile:90.69(Chemistry, Physical)Falyouna, O.*; Maamoun, I.; Ghosh, S.*; Malloum, A.*; Othmani, A.*; Eljamal, O.*; Amen, T. W. M.*; Oroke, A.*; Bornman, C.*; Ahmadi, S.*; et al.
Journal of Molecular Liquids, 368, Part B, p.120726_1 - 120726_25, 2022/12
Times Cited Count:5 Percentile:42.74(Chemistry, Physical)Yamaguchi, Toshio*; Yoshida, Koji*; Machida, Shinichi*; Hattori, Takanori
Journal of Molecular Liquids, 365, p.120181_1 - 120181_10, 2022/11
Times Cited Count:1 Percentile:15.15(Chemistry, Physical)Neutron scattering measurements were performed on an aqueous 3 mol/kg NaCl solution in D O at temperature and pressure conditions of 0.1 MPa/298K, 1 GPa/298K, 1 GPa/523K, and 4 GPa/523K. The empirical potential structure refinement method was applied to the obtained data to extract the pair correlation function, coordination number distribution, angular distribution (orientation correlation), and spatial density function (3-D structure). From those results, pressure and temperature dependence of solvation and association of ions and solvent-water structure were discussed.
O at temperature and pressure conditions of 0.1 MPa/298K, 1 GPa/298K, 1 GPa/523K, and 4 GPa/523K. The empirical potential structure refinement method was applied to the obtained data to extract the pair correlation function, coordination number distribution, angular distribution (orientation correlation), and spatial density function (3-D structure). From those results, pressure and temperature dependence of solvation and association of ions and solvent-water structure were discussed.
 /water nanofluid
/water nanofluidYoshida, Koji*; Sanada, Yusuke*; Yamaguchi, Toshio*; Matsuura, Masato*; Tamatsukuri, Hiromu; Uchiyama, Hiroshi*
Journal of Molecular Liquids, 366, p.120218_1 - 120218_9, 2022/11
Times Cited Count:1 Percentile:15.15(Chemistry, Physical)Falyouna, O.*; Idham, M. F.*; Maamoun, I.; Bensaida, K.*; Ashik, U. P. M.*; Sugihara, Yuji*; Eljamal, O.*
Journal of Molecular Liquids, 359, p.119323_1 - 119323_20, 2022/08
Times Cited Count:37 Percentile:99.49(Chemistry, Physical)Maamoun, I.; Bensaida, K.*; Eljamal, R.*; Falyouna, O.*; Tanaka, Kazuya; Tosco, T.*; Sugihara, Yuji*; Eljamal, O.*
Journal of Molecular Liquids, 358, p.119216_1 - 119216_13, 2022/07
Times Cited Count:27 Percentile:98.75(Chemistry, Physical)Zhang, W. Q.*; Yamaguchi, Toshio*; Fang, C. H.*; Yoshida, Koji*; Zhou, Y. Q.*; Zhu, F. Y.*; Machida, Shinichi*; Hattori, Takanori; Li, W.*
Journal of Molecular Liquids, 348, p.118080_1 - 118080_11, 2022/02
Times Cited Count:2 Percentile:32.24(Chemistry, Physical)The ion hydration and association and hydrogen-bonded water structure in an aqueous 3 mol/kg RbCl solution were investigated at 298 K/0.1 MPa, 298 K/1 GPa, 523 K/1 GPa, and 523 K/4 GPa by neutron diffraction combined with EPSR methods. The second hydration layer of Rb and Cl
and Cl becomes evident under elevated pressure and temperature conditions. The average oxygen coordination number of Rb
becomes evident under elevated pressure and temperature conditions. The average oxygen coordination number of Rb (Cl
(Cl ) in the first hydration layer increases from 6.3 (5.9) ambient pressure to 8.9 (9.1) at 4 GPa, while decreasing coordination distance from 0.290 nm (0.322 nm) to 0.288 nm (0.314 nm). The orientation of the water dipole in the first solvation shell of Rb
) in the first hydration layer increases from 6.3 (5.9) ambient pressure to 8.9 (9.1) at 4 GPa, while decreasing coordination distance from 0.290 nm (0.322 nm) to 0.288 nm (0.314 nm). The orientation of the water dipole in the first solvation shell of Rb and a central water molecule is sensitive to pressure, but that in the first solvation shell of Cl
and a central water molecule is sensitive to pressure, but that in the first solvation shell of Cl does not change very much. The number of contact-ion pairs Rb
does not change very much. The number of contact-ion pairs Rb -Cl
-Cl decreases with elevated temperature and increases with elevated pressure. Water molecules are closely packed, and the tetrahedral hydrogen-bonded network of water molecules no longer exists in extreme conditions.
decreases with elevated temperature and increases with elevated pressure. Water molecules are closely packed, and the tetrahedral hydrogen-bonded network of water molecules no longer exists in extreme conditions.
Abe, Hiroshi*; Nemoto, Fumiya*; Hiroi, Kosuke; Oishi, Kazuki*; Takata, Shinichi
Journal of Molecular Liquids, 346, p.117035_1 - 117035_6, 2022/01
Times Cited Count:4 Percentile:58.62(Chemistry, Physical)Hashimoto, Shunsuke*; Nakajima, Kenji; Kikuchi, Tatsuya*; Kamazawa, Kazuya*; Shibata, Kaoru; Yamada, Takeshi*
Journal of Molecular Liquids, 342, p.117580_1 - 117580_8, 2021/11
Times Cited Count:3 Percentile:25.84(Chemistry, Physical)Quasi-elastic neutron scattering (QENS) and pulsed-field-gradient nuclear magnetic resonance (PFGNMR) analyses of a nanofluid composed of silicon dioxide (SiO ) nanoparticles and a base fluid of ethylene glycol aqueous solution were performed. The aim was to elucidate the mechanism increase in the thermal conductivity of the nanofluid above its theoretical value. The obtained experimental results indicate that SiO
) nanoparticles and a base fluid of ethylene glycol aqueous solution were performed. The aim was to elucidate the mechanism increase in the thermal conductivity of the nanofluid above its theoretical value. The obtained experimental results indicate that SiO particles may decrease the self-diffusion coefficient of the liquid molecules in the ethylene glycol aqueous solution because of their highly restricted motion around these nanoparticles. At a constant temperature, the thermal conductivity increases as the self-diffusion coefficient of the liquid molecules decreases in the SiO
particles may decrease the self-diffusion coefficient of the liquid molecules in the ethylene glycol aqueous solution because of their highly restricted motion around these nanoparticles. At a constant temperature, the thermal conductivity increases as the self-diffusion coefficient of the liquid molecules decreases in the SiO nanofluids.
nanofluids.
 -lactoglobulin
-lactoglobulinYoshida, Koji*; Zenin, Tomohiro*; Fujiyoshi, Ayako*; Sanada, Yusuke*; Yamaguchi, Toshio*; Murata, Kunihiko*; Takata, Shinichi; Hiroi, Kosuke; Takahiro, Takekiyo*; Yoshimura, Yukihiro*
Journal of Molecular Liquids, 293, p.111477_1 - 111477_9, 2019/11
Times Cited Count:8 Percentile:43.54(Chemistry, Physical)Abe, Hiroshi*; Yamada, Takeshi*; Shibata, Kaoru
Journal of Molecular Liquids, 264, p.54 - 57, 2018/08
Times Cited Count:14 Percentile:60.05(Chemistry, Physical)Okamoto, Yoshihiro; Osugi, Takeshi; Akabori, Mitsuo; Kobayashi, Toru; Shiwaku, Hideaki
Journal of Molecular Liquids, 232, p.285 - 289, 2017/04
Times Cited Count:3 Percentile:12.07(Chemistry, Physical)High energy XAFS measurement using cerium K-edge was performed to study the chemical state of cerium in high-temperature molten slag (SiO -CaO-Fe
-CaO-Fe O
O -CeO
-CeO ). It was found from the change in the nearest Ce-O distance obtained from EXAFS analysis and the energetic shift of the white line peak observed in XANES analysis that oxidation state of cerium was tetravalent in the molten state and trivalent in solid state. The Debye-Waller factor of the nearest Ce-O pair in solid slag was very large even at room temperature, and the change in its value upon heating and melting was very small. This result suggests that cerium is highly disordered and stable in solid slag.
). It was found from the change in the nearest Ce-O distance obtained from EXAFS analysis and the energetic shift of the white line peak observed in XANES analysis that oxidation state of cerium was tetravalent in the molten state and trivalent in solid state. The Debye-Waller factor of the nearest Ce-O pair in solid slag was very large even at room temperature, and the change in its value upon heating and melting was very small. This result suggests that cerium is highly disordered and stable in solid slag.
Murakami, Hiroshi
Journal of Molecular Liquids, 210, Part A, p.37 - 43, 2015/10
Times Cited Count:10 Percentile:38.38(Chemistry, Physical)Picosecond relaxation dynamics of water before and after water shedding from reverse micelles are studied using temperature-dependent terahertz time-domain spectroscopy measurements to clarify the confinement effect of the reverse micelle on the dynamics of water. Before water shedding, the relaxation process due to the collective motion of bulk-like water in the core of the reverse micelle exhibits a considerable slowing down in comparison with that in liquid water as the temperature decreases. This is attributed to the confinement effect of reverse micelles. Moreover, the time constant abruptly drops at the water shedding, and its temperature behavior is similar to that of liquid water afterwards. These results demonstrate that the water extracted from the reverse micelle attains the property of liquid water by reduction in the confinement imposed on the motion of the water.
 systems
systemsOkamoto, Yoshihiro; Shiwaku, Hideaki; Yaita, Tsuyoshi; Suzuki, Shinichi; Gaune-Escard, M.*
Journal of Molecular Liquids, 187, p.94 - 98, 2013/11
Times Cited Count:14 Percentile:47.51(Chemistry, Physical)The local structure of molten GdCl and its mixture in LiCl-KCl eutectic was investigated by Gd K-edge extended X-ray absorption fine structure (EXAFS) technique. As the result of curve fitting analysis, the nearest Gd
and its mixture in LiCl-KCl eutectic was investigated by Gd K-edge extended X-ray absorption fine structure (EXAFS) technique. As the result of curve fitting analysis, the nearest Gd -Cl
-Cl distance was 2.74
distance was 2.74 0.02
0.02 with coordination number 6.5
with coordination number 6.5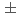 0.3 in the pure melt. On the other hand, those in the 10%GdCl
0.3 in the pure melt. On the other hand, those in the 10%GdCl mixture melt decreased to 2.70
mixture melt decreased to 2.70 0.01
0.01 and 6.1
and 6.1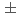 0.3, respectively. These results suggest that octahedral coordination (GdCl
0.3, respectively. These results suggest that octahedral coordination (GdCl )
) structure is formed and stabilized by mixing with the alkali chlorides. The result was compared with that of YCl
structure is formed and stabilized by mixing with the alkali chlorides. The result was compared with that of YCl and LaCl
and LaCl in LiCl-KCl eutectic melt.
in LiCl-KCl eutectic melt.
Yamaguchi, Toshio*; Fujimura, Koji*; Uchi, Kazuya*; Yoshida, Koji*; Katayama, Yoshinori
Journal of Molecular Liquids, 176, p.44 - 51, 2012/12
Times Cited Count:18 Percentile:55.43(Chemistry, Physical)X-ray diffraction measurements in an energy-dispersive mode have been made on water under various thermodynamic conditions of 298 K/30 MPa, 473 K/30 MPa and 573 K/30 MPa on a laboratory X-ray diffractometer. All the X-ray structure factors as well as those of water already measured at 298 K/1 GPa, 473 K/0.35 GPa and 486 K/4 GPa by using synchrotron X-ray radiation were subjected to empirical potential structure refinement (EPSR) modeling to reveal the detailed hydrogen bonding features in terms of partial pair correlation function, coordination number and three-dimensional spatial density function as a function of temperature and pressure. It has been shown to what extent the tetrahedral hydrogen-bonded network of water is perturbed by pressure and temperature.
 Pb
Pb intermetallic compound
intermetallic compoundOno, Masao; Huang, X. S.*; Shibata, Yasuhiro*; Iguchi, Yusuke*; Sakai, Seiji; Maekawa, Masaki; Chen, Z. Q.*; Osakabe, Toyotaka; Kawasuso, Atsuo; Naramoto, Hiroshi*; et al.
Proceedings of 1st International Conference on Diffusion in Solids and Liquids (DSL 2005), p.531 - 533, 2005/07
Recently, we formed atomic-scale graded structures in some miscible alloys and observed the decomposition in Bi Pb
Pb intermetallic compound by sedimentation of atoms under strong gravitational field. In this study, we measured positron lifetime of centrifuged Bi
intermetallic compound by sedimentation of atoms under strong gravitational field. In this study, we measured positron lifetime of centrifuged Bi Pb
Pb , to which the composition change was very small as it was treated at low temperature. It was found that the positron lifetime became longer than that of starting state. This indicated that the point defects (vacancy or divacancy) increased in the sample by centrifugal treatment. We are now investigating the relationship between increase in point defects and sedimentation of atoms.
, to which the composition change was very small as it was treated at low temperature. It was found that the positron lifetime became longer than that of starting state. This indicated that the point defects (vacancy or divacancy) increased in the sample by centrifugal treatment. We are now investigating the relationship between increase in point defects and sedimentation of atoms.
Murakami, Hiroshi
Journal of Molecular Liquids, 89(1-3), p.33 - 45, 2000/12
Times Cited Count:9 Percentile:27.42(Chemistry, Physical)no abstracts in English
Tsuji, Kazuhiko*; Katayama, Yoshinori
Physics of Complex Liquids, p.83 - 97, 1998/00
Structural changes with pressure for liquid Se and liquid Te have been studies. X-ray diffraction and extended x-ray absorption fine structure (EXAFS) have been measured at pressure up to 8 GPa by using a cubic-type multianvil apparatus and synchrotron radiation. With increasing pressure, the interchain atomic distance becomes short and approaches to the interchain atomic distance in liquid Se. In metallic liquid Se, two kinds of bonds coexist; shot bond and lond bond. The fraction of short bond decreases with increasing pressure, indicating weakening and breaking of covalent bonds. The structure of liquid Se under high pressure is similar to that of metallic liquid Te at atmospheric pressure. These results are discussed in connection with semiconductor-to-metal transitions in liquid Se, liquid Te and liquid Se Te
Te alloys with pressure, temperature and concentration.
alloys with pressure, temperature and concentration.